The use and combination of various metals in water and sewage installations can result in galvanic corrosion and very high corrosion rates. In this article, the authors present the general principles of galvanic corrosion with some case studies and solutions.
The standard approach to corrosion prevention is to use coatings and corrosion-resistant materials. These measures are usually satisfactory for the actual structure. It may, however, have an unfavorable effect on neighboring structures.
At present, a wide variety of materials is available for water and wastewater installations. However, combinations of these might, under certain conditions, lead to severely accelerated galvanic corrosion and cause damage within a short time. These effects are highly relevant for new buildings – and extensions or renovations to existing water installations in particular.
While the principal issues of galvanic corrosion are well understood, appropriate preventative measures are seldom adequately applied. In addition, the specific conditions and type of installation typically exercise a considerable influence on the corrosion rate.
The principle of corrosion
Corrosion is usually an electrochemical interaction between a metal and its environment, where the properties of the metal change and thus also its functionality, environment, or technical system.
Electrochemical corrosion is mainly an anodic oxidation reaction, where the metal atom is dissolved as a positively charged ion while releasing electrons according to equation (1) and the cathodic oxygen reduction in equation (2):
- Fe → Fe2+ + 2e-
- O2 + 2 H2O + 4 e– → 4 OH-
To satisfy the condition of electroneutrality, the number of electrons used for each reaction at any time must be equal. This relation is schematically depicted in Figure 1, where the size of the arrows represents the rate of the corresponding reaction. The anodic process is shown as red arrows, while blue arrows indicate the cathodic process.
On certain metals, the formation of a protective layer of oxides can lead to passivity. In this case, the anodic corrosion process is decreased to a negligibly low level. An example of a passive metal in water systems is stainless steel.
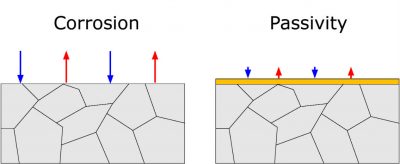
Figure 1: Electrochemical reactions occurring on the metal surface under different conditions
An electrochemical potential is established at the metal’s interface with an electrolyte (e.g., water). This ‘corrosion potential’ depends on the material, the concentrations of dissolved oxygen and the metal ions in the electrolyte, as well as the formation of passive and other protective layers. Table 1 below gives a few examples of the corrosion potentials of different metals in water.
| Metal | Corrosion potential [VCSE] |
| Copper | -0.10 |
| Stainless steel | -0.10 |
| Red-bronze and Si-bronze | -0.20 |
| Brass | -0.35 |
| Iron in aerated water | -0.55 |
| Iron in stagnant water | -0.75 |
| Galvanized steel | -1.0 |
Table 1: Examples of corrosion potentials of different metals in water
Where there is an electrically conductive connection between two metals with different corrosion potentials, a galvanic cell is established. The reduction of oxygen occurs on both metal surfaces, while the oxidation reaction takes place primarily on the metal exhibiting the most negative potential, resulting in the accelerated dissolution of the metal. The geometric effect plays an important role since a small anode in contact with a large cathode can lead to corrosion rates up to 2mm/year in typical water applications.
Examples of unfavorable material combinations are shown in Figure 2 below.
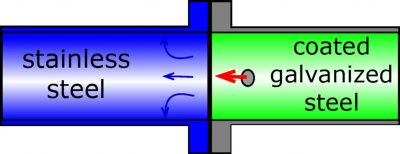
Figure 2a) Combination of stainless steel and galvanized steel with an internal coating.
Simultaneous electric and electrolytic contact between stainless and galvanized steel will establish a galvanic element that accelerates the corrosion rate of the galvanized steel due to the increase in current (Figure 2a).
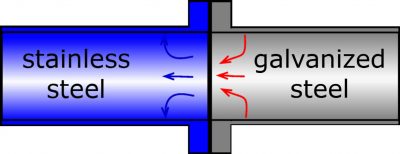
Figure 2b) Combination of stainless steel and galvanized steel without an internal coating.
An internal coating such as a synthetic resin applied to the galvanized steel might even increase local corrosion since the entire anodic process is confined to the defect in the coating, thereby leading to a high local corrosion rate (Figure 2b).
Mitigation of galvanic corrosion
The following two solutions can be applied to minimize the risk of galvanic corrosion:
Galvanic separation
Electrical separation of material is possible to mitigate galvanic corrosion by using insulating flanges or muffs to interrupt the electric current. In practice, these insulations are, unfortunately, often bypassed, either for reasons of safety (equipotential bonding) or by accident.
The use of insulating flanges or muffs is thus often only a feasible solution to separate single devices, for example, a water meter made from cast iron and internally coated, which is then integrated into a stainless steel pipeline. The water meter is electrically isolated from the system and will only suffer from galvanic corrosion if it is connected to the equipotential bonding.
Insulation
The other method to reduce the effects of a galvanic cell is by increasing the electric resistance in the electrolyte, for example, by incorporating an electrically insulating section between the related metal parts.
This can, for example, be a plastic pipe between the stainless steel pipe and the galvanized steel pipe or an internal coating of the stainless steel pipe (the cathode) as well as on the flange.
The length of the insulating section required depends on water conductivity, pipe diameters, and the potential difference between the metals. In drinking water installations, a length of at least five times the pipe diameter is typically required.
Additional findings
Water composition
Experience has repeatedly shown that the formation of a thin, insulating layer of calcium carbonate, which is deposited on the metal with the most positive corrosion potential acting as a cathode, reduces the risk of galvanic corrosion. The formation of this layer is favored when a pH increase occurs at the cathode as the result of oxygen reduction, as seen in reaction (2) above, which affects the lime-carbonic acid equilibrium so that precipitation of calcium carbonate occurs.
Use of red-bronze fittings
It has been common to use red-bronze elements between sections of stainless steel and galvanized steel pipe. This might decrease the potential difference of the galvanic cell (Table 1) and thereby reduce the corrosion rate. However, the experience of corrosion-related deterioration in such cases has shown that this approach is not considered a reliable method of corrosion protection, particularly when no insulating layer can be formed on the red-bronze.
The alloy composition of stainless steel
A variety of stainless steel alloys are admitted to be used for water installations. Electric contacts between them do not accelerate corrosion, even in the case of different corrosion potentials. The passive protective layer prevents corrosion and mitigates the galvanic cell.
Galvanic corrosion can cause severe damage to water and sewage installations. The growing number of different materials being used increases the risk of corrosion. This is especially true when replacing parts of the existing piping made of galvanized steel.
The presented mitigation measures allow for eliminating the problem of galvanic corrosion without compromising the safety of electrical installations.

